Argon plasma ionization in thermodynamic equilibrium with continuity equation
DOI:
https://doi.org/10.59190/stc.v5i3.318Keywords:
Argon, Continuity, Density, Equilibrium, PlasmaAbstract
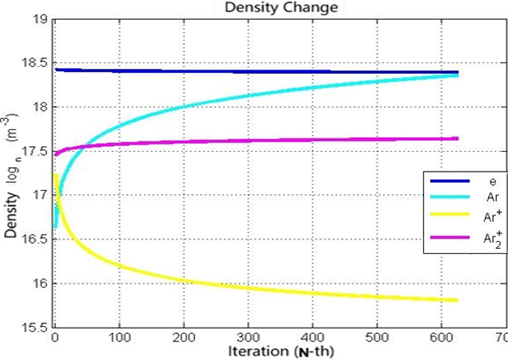
Local thermodynamic equilibrium is a foundational concept in plasma physics and heat transfer, describing a state where each small region of a system can be treated as if it is in thermodynamic equilibrium, even if the whole system is not. However, achieving accurately perfect thermodynamic equilibrium conditions in real-experiments is often challenging. It often struggles for understanding phenomena like excited states or specific Arrhenius-driven reactions. As a result, the advantages of plasma modeling with simplifications can sometimes overshadow the disadvantages of experiments. This study simulated the ionization process of argon plasma using the 4th order Runge-Kutta numerical method. The simulation, initiated with initial densities before the simulation is run, each of them is electrons 2.6 × 1018 m-3, neutral argon (Ar) 2.6 × 1018 m-3, positive argon ions (Ar+) 2.6 × 1018 m-3, and positive diatomic argon ions (Ar2+) 2.6 × 1018 m-3, successfully obtained reaction rate equilibrium data at the 625th iteration. The final densities observed were 2.46 × 1018 m-3 for electrons, 2.27 × 1018 m-3 for neutral argon, 6.4 × 1015 m-3 for Ar+, and 4.34 × 1017 m-3 for Ar2+. These results show the equilibrium reaction rate in argon plasma which provides information that density of electron and Ar+ species show a decreasing trend while density of Ar and Ar2+ species shows an increasing trend which are the result of ionization and recombination processes in the entire plasma system.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Anshori Kasri, Saktioto Saktioto, Rakhmawati Farma, Ari Sulistyo Rini, Erwin Erwin, Awitdrus Awitdrus

This work is licensed under a Creative Commons Attribution 4.0 International License.










